What is the cause of peak tailing?
Peak tailing was attributed to problems related to chemical interactions on the column. One way of thinking about peak tailing is that the active sites — the places on the column where interactions between the analyte molecules and the chemical surface of the column occur — become saturated.
What causes peak tailing in ion chromatography?
The primary cause of peak tailing is the occurrence of more than one mechanism of analyte retention. In reversed-phase separations, analyte retention is usually achieved through nonspecific hydrophobic interactions with the stationary phase.
What is the tailing factor of fronting?
Using the USP Tailing formula, we could calculate fronting by the following: width @5% of peak height/(2 x back half of the peak) (figure 3). Alternatively we could use the symmetry calculation which is back half/front half and flip that around to be front half/back half (figure 4).
What is the cause of peak splitting in HPLC?
A Blocked Frit The frit is a key component of the HPLC system that keeps the packing material inside the column while ensuring the sample flow remains consistent and uniform. A blockage in the column frit can disrupt the flow path of the analyte, altering retention time and causing peak splitting.
What causes peak fronting?
What is Peak Fronting in Chromatography? Peak fronting occurs when an asymmetric peak is broader in the first half and narrower in the second half. Several factors may cause peak fronting including poor sample solubility, column collapse, or saturation/overload of the column.
How to prevent peak tailing?
When you know you will be working with basic analytes, choosing end-capped columns is a proactive method of preventing peak tailing due to secondary interactions. Using columns with high-purity silica is also advisable to prevent interactions between analytes and contaminants such as trace metals.
What is peak tailing and fronting in chromatography?
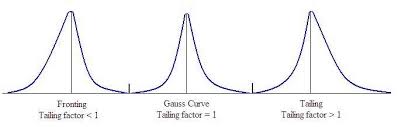
Tailing is basically the inverse of fronting. The peak is presented asymmetrically, with a broader second half and a narrower first half – breaking away from the ideal peak shape, with its symmetrical Gaussian profile. While the effect is similar, the circumstances of tailing are different from those of fronting.
How to remove fronting in HPLC?
Volume overloading-Injecting too large of a volume can result in fronting, since it broadens the peak. You can eliminate this possibility by injecting a smaller volume.
How to reduce peak tailing in GC?
Trimming the end off of a column can help remove activity at the end of the column. If this does not work, you may need to replace your column. Column blockage may cause peak tailing. Inject something with no known tailing to test if a blockage is present.
What causes tailing in TLC?
Tailing in TLC; is due to the incorrect solvent system moving phase. Some of the compounds such as amine gives tailing. You can solve it by adding a few drops of triethylamine in the solvent mixture.
How to improve tailing factor?
Filtration of the samples prior to introduction on the column can help prevent peak tailing resulting from collection of particles at the inlet frit. Possible solutions: You could try using a bigger column or decreasing the sample amounts introduced to the column to eliminate the problem.
What causes peak splitting in GC?
Split peaks can show up in gas chromatography when our injection isn’t working right or things aren’t transferring from the inlet to the column correctly.
What causes peak tailing in GC chromatogram?
Figure 1: Capillary GC chromatogram in which all analytes and the injection solvent peak show tailing to a greater or lesser degree. Sometimes peak tailing can be caused by chemical interactions between certain analytes (usually more polar) and surfaces within the GC system.
How to fix peak fronting HPLC?
Solution: Inject smaller volume or dilutions (e.g. 1:10 or 1:100) of sample.
What causes NMR peak splitting?
Spin splitting in NMR refers to the phenomenon where a single absorption peak is split into multiple peaks, such as doublets, triplets, or quartets, due to the influence of neighboring protons.
What causes peak tailing in HPLC?
What Factors Affect HPLC Peak Tailing? Ionized silanols will ion-exchange with protonated bases which can cause tailing and method variability. This occurs most often at mid pH where silanols are ionized. Unprotonated acids can compete for H+ with protonated silanols; this can occur at low pH.
How to prevent peak fronting?
As the sample doesn’t travel through the column in a tight band, the peak is broadened and increased separation times lead to peak fronting. There are two simple methods to fix this problem: Reduce the solute concentration in the sample. Reduce the injection volume of the sample.
What causes peak splitting in HPLC?
Blocked frit may cause tailing or it may cause split peak. A blocked frit can cause the fraction of the sample to spread on the surface of the column faster and the part of the sample is delayed and this causes Peak Splitting.
What is peak tailing vs fronting?
The chromatographic peak in (a) is an example of tailing, which occurs when some sites on the stationary phase retain the solute more strongly than other sites. The peak in (b) is an example of fronting, which most often is the result of overloading the column with sample.
What is an acceptable peak tailing?
Acceptable Tailing A new column is considered acceptable if the As value is 0.9 – 1.2 (0.9 indicates slight fronting). In practical terms, an As value below 1.5 is usually OK to work with, and up to As = 2.0 may be acceptable depending on the separation and resolution of the peaks.
How does tailing occur?
Tailings are a by-product of mining. After ore containing an economically-recoverable commodity is mined from the earth, that commodity is extracted in a processing plant or mill. After the commodity of value is extracted from the ore material, the resultant waste stream is termed “tailings”.
How to avoid tailing in HPLC?
If compounds are basic you can add 0.1 % of diethyl amine. If they are acidic then you can add 0.1 % trifluoroacetic acid. Both will prevent tailing by avoiding interaction with free OH in the stationary phase. Try cleaning the guard column and increasing the flow rate through the column.
What is the limit for tailing factor in HPLC?
T should be less than or equal to 2 to satisfy the system suitability requirement. The tailing factor in HPLC is also known as the symmetry factor.
What causes fronting peaks in GC?
In many cases the cause of peak fronting is an overload of the GC column produced by the injection of too great a mass of analyte.
How to fix peak fronting in GC?
We have a few different options to correct the problem. We can put less material on the column, choose a column with a larger ID, choose a column with a thicker film, or choose a stationary phase that is more compatible with our sample.
Why is tailing fronting behaviour in process chromatograms undesired?
There are many different causes to “fronting” or “tailing” peaks, but most can be easily remedied. For example, fronting peaks are often caused by column overload or overpacking. Similarly, tailing peaks can be caused by underpacking, or by having a sample that is too viscous.
What causes peak tailing in GC MS?
Tailing peaks are usually created through turbulence within the hydraulic path of the carrier gas as it passes through the system. They may also be caused by unswept volumes within the system.
What causes tailing on a TLC plate?
Tailing in TLC; is due to the incorrect solvent system moving phase. Some of the compounds such as amine gives tailing. You can solve it by adding a few drops of triethylamine in the solvent mixture.
How does tailing occur?
Tailings are a by-product of mining. After ore containing an economically-recoverable commodity is mined from the earth, that commodity is extracted in a processing plant or mill. After the commodity of value is extracted from the ore material, the resultant waste stream is termed “tailings”.
What is the tailing factor of peak asymmetry?
The peak asymmetry is defined as the tail width (b) / front width (a). This can alternatively be calculated as the Tailing Factor using the formula T=(a+b)/2a.
What causes fronting and tailing peaks?
What causes peak tailing?
What causes peak fronting?
What is the difference between fronting and tailing?
Hey there, chromatography enthusiasts! Today, we’re diving into the fascinating world of peak tailing and fronting, two common phenomena that can throw a wrench in your chromatography analysis.
Imagine you’re running a chromatography experiment, and instead of getting those nice, symmetrical peaks, you end up with peaks that look like they’ve been stretched out or compressed. Peak tailing happens when the trailing edge of the peak is broader than the leading edge, resulting in a peak with a longer tail. On the other hand, peak fronting occurs when the leading edge of the peak is broader than the trailing edge, creating a peak with a shorter tail.
These distortions can significantly impact the accuracy of your results, especially when quantifying compounds. So, let’s get to the root of the problem and explore the common causes of peak tailing and fronting.
Unveiling the Culprits of Peak Tailing
Think of peak tailing as a symptom of a deeper problem within your chromatographic system. Here are some of the most common suspects:
Injection Volume: Just like overfilling a glass of water, injecting too large a sample volume can lead to peak tailing. The analyte molecules get spread out across the stationary phase, resulting in a broader peak. Always ensure your injection volume is within the recommended range for your system and column.
Column Overloading: This occurs when you inject a sample concentration that exceeds the column’s capacity to retain and separate the analyte. Think of it as having too many people trying to fit on a small bus—things start getting cramped and messy. Using a smaller injection volume or a more dilute sample can help overcome this issue.
Active Sites: Imagine your stationary phase as a surface with pockets that can interact with your analyte molecules. Active sites are imperfections or irregularities on the stationary phase that can cause strong interactions with the analyte, leading to peak tailing. These active sites can be caused by impurities in the stationary phase, residual manufacturing chemicals, or even damage to the column.
Sample Matrix Effects: Sometimes, the sample itself can wreak havoc on your analysis. The sample matrix refers to the other components present in the sample besides the analyte of interest. These components can interact with the stationary phase and interfere with analyte separation, leading to peak tailing.
Column Age: Just like anything else, chromatography columns wear out over time. As the column ages, the stationary phase may degrade, leading to increased peak tailing. Regularly assess your column’s performance and consider replacing it when necessary.
Column Temperature: Temperature plays a crucial role in chromatography. If the column temperature is too low, the analyte molecules may interact with the stationary phase too strongly, leading to peak tailing. Optimize your column temperature for optimal separation.
Mobile Phase Composition: The mobile phase acts as the solvent that carries your analytes through the column. Changes in the mobile phase composition can alter the separation process, leading to peak tailing. Experiment with different mobile phases and optimize the composition to achieve the desired separation.
Flow Rate: The flow rate of the mobile phase determines how quickly the analyte molecules travel through the column. A flow rate that’s too high can lead to peak broadening, including peak tailing. Optimize the flow rate for your specific application.
Detector Settings: The detector picks up the signals from the separated analytes. Improper detector settings, such as a too-high sensitivity, can lead to peak tailing. Ensure your detector is properly calibrated and the settings are appropriate for your analysis.
Tackling Peak Fronting: A Guide to Peak Symmetry
Now let’s shift gears and address peak fronting. Peak fronting often suggests an issue with the injection process or the interaction between the analyte and the stationary phase. Here are the primary causes of peak fronting:
Injection Technique: The way you inject your sample can significantly impact peak shape. An uneven injection, where the sample doesn’t distribute evenly within the injection port, can lead to peak fronting. Ensure consistent and accurate injection techniques to minimize this problem.
Sample Concentration: If the concentration of your sample is too high, it can overwhelm the column’s capacity to retain and separate the analyte. This can result in peak fronting, as the leading edge of the peak elutes faster than the trailing edge. Diluting the sample can often solve this issue.
Mobile Phase pH: The pH of the mobile phase can greatly influence the interaction between the analyte and the stationary phase. If the pH is not optimal, the analyte may interact with the stationary phase in a way that promotes peak fronting. Optimize the mobile phase pH for your specific application.
Strong Interactions: Sometimes, your analyte may have a very strong affinity for the stationary phase. These strong interactions can lead to peak fronting, as the analyte molecules get stuck at the beginning of the column, resulting in a narrow leading edge and a broader trailing edge. Adjusting the mobile phase composition or using a different column with a less polar stationary phase can help reduce these interactions.
Column Degradation: Just like with peak tailing, column degradation can contribute to peak fronting. Over time, the stationary phase can become less efficient, leading to reduced retention and peak fronting. Consider replacing your column if it’s no longer performing as expected.
Mastering Chromatography: Peak Optimization Techniques
Now that we’ve explored the common causes of peak tailing and fronting, let’s equip ourselves with some tips and tricks to optimize peak shape:
Method Development: Start by carefully developing your chromatographic method, including selecting the appropriate column, mobile phase, and temperature. Consider the properties of your analytes and the desired separation.
System Optimization: Regularly check and optimize your chromatographic system, including the injection port, detector, and column. Ensure everything is working properly and that the settings are appropriate for your analysis.
Sample Preparation: Proper sample preparation is crucial for achieving good peak shape. Remove any contaminants or interfering compounds that might affect the separation process. Consider using appropriate sample preparation techniques like filtration, extraction, or derivatization.
Column Conditioning: Before running your samples, condition your column properly to ensure optimal performance. This involves equilibrating the column with the mobile phase and establishing a stable baseline.
Calibration: Calibrate your system with known standards to ensure the accuracy of your results. Regular calibration helps identify and correct any system drifts or inconsistencies that might affect peak shape.
Troubleshooting: If you encounter peak tailing or fronting, don’t panic! Systematically troubleshoot the problem by carefully considering the possible causes and making adjustments to your method or system as needed.
FAQs: Your Chromatography Questions Answered
Let’s address some common questions you might have about peak tailing and fronting:
1. Can I improve peak shape without changing my method?
While optimizing your method is often the best approach, there are some adjustments you can make without completely changing your method. You can try:
Reducing your injection volume.
Increasing the column temperature.
Adjusting the flow rate.
2. Why do my peaks look asymmetric, but not exactly like tailing or fronting?
Asymmetrical peaks can have various causes. One possibility is peak distortion, which can be caused by issues with the detector, the signal processing, or the data analysis.
3. What are the best resources for learning more about peak optimization?
There are numerous resources available, including online courses, textbooks, and scientific journals. You can also find helpful information and discussions on forums dedicated to chromatography.
4. Can I completely eliminate peak tailing or fronting?
It’s often impossible to completely eliminate peak tailing or fronting, but you can significantly minimize it by carefully optimizing your chromatographic system and method.
5. Is there a universal solution for peak tailing and fronting?
Unfortunately, there’s no one-size-fits-all solution. The best approach is to identify the specific cause of the problem and take appropriate steps to address it.
6. How can I tell if peak tailing or fronting is due to my method or my system?
This can be tricky. A good starting point is to test different methods and systems. If the issue persists across different methods and systems, it might indicate a problem with your sample or the way you’re injecting it.
7. Is there a way to analyze my data even if I have peak tailing or fronting?
You can still analyze your data, but the accuracy of your results might be compromised. Software can be used to deconvolute or correct for peak tailing, but these methods have limitations.
8. What are the implications of peak tailing and fronting on my results?
Peak tailing and fronting can lead to inaccurate quantification, as the peak area used for quantitation might be underestimated or overestimated. It can also affect the resolution of your separation, making it difficult to distinguish between closely eluting peaks.
9. I’m new to chromatography. Where should I start?
Start with the basics! Learn about the different types of chromatography, the principles of separation, and the common causes of peak tailing and fronting. Practice your techniques and experiment with different methods to gain experience.
10. Can I fix peak tailing and fronting with software?
Software can be used to correct for peak tailing or fronting, but it’s not a perfect solution. It’s best to address the root cause of the problem for the most accurate results.
By understanding the causes and learning how to optimize peak shape, you can ensure accurate and reliable results from your chromatography experiments. Good luck and happy chromatographing!
See more here: What Causes Peak Tailing In Ion Chromatography? | Causes Of Peak Tailing And Fronting
Understanding Chromatogram Peaks – Fronting, Tailing,
Peak Tailing. Tailing is basically the inverse of fronting. The peak is presented asymmetrically, with a broader second half and a narrower first half – Chemtech International
What is Peak Fronting? | PerkinElmer
The good news is that there are a few typical causes of peak fronting: Overloading. Co-elution. Mismatch between the sample and the chromatographic system. Column perkinelmer.com
Troubleshooting Basics, Part IV: Peak Shape Problems
However, there are several other potential causes of peak tailing (or fronting) as well, so it is a good idea to track the peak shape over time to anticipate Chromatography Online
How to fix asymmetrical chromatography peak | Cytiva
There are many different causes to “fronting” or “tailing” peaks, but most can be easily remedied. For example, fronting peaks are often caused by column overload or Cytiva
Peak Fronting in GC – Articles – GC Portal – Agilent Community
Peak fronting is present in a chromatography peak when it has an excessive asymmetry with a leading edge. A normal peak is almost symmetrical. Other Agilent Community
What is Peak Fronting? – Chromatography Today
The aim in high performance liquid chromatography (HPLC) and gas chromatography (GC) is to get good peak shape and good separation. The most desirable outcome is the generation of symmetric Chromatography Today
A Tail of Two Peaks: Troubleshooting Poor Peak Shape – Agilent
1: Common Peak Shape Issues. Peak tailing – flow path or activity. Bonus peaks – in sample or back flash (carry-over) Split peaks – injector problems, mixed solvent. No Agilent
Peak Tailing in HPLC – Crawford Scientific
Peak tailing is the most common chromatographic peak shape distortion. We want to address how to go about fixing these distortions but first, let’s understand what causes peak tailing. Peak Element
Peak Tailing in GC Systems – Articles – GC Portal – Agilent
Peak tailing is present in a chromatography peak when it has an excessive asymmetry with a trailing edge. A normal peak is almost symmetrical. Other chromatographic problems Agilent Community
See more new information: charoenmotorcycles.com
Front Tailing Peaks Aka Peak Fronting – How To Reduce And Troubleshoot In Hplc And Gc
Lc Troubleshooting—All Of My Peaks Are Tailing! What Should I Do?
Tailing And Fronting Of Peaks In Chromatograms And Reasons For Peak Distortion
Hplc Tips Peak Tailing
Hplc Trouble Shooting|Peak Shape Issue|Peak Tailing|Peak Fronting
Gc Troubleshooting—Tailing Peaks
Tailing Dan Fronting
Separations: Overloading (Fronting) \U0026 Tailing
Peak Tailing: Phenomenon, Symptoms, And Corrections
Link to this article: causes of peak tailing and fronting.
See more articles in the same category here: https://charoenmotorcycles.com/how