What is the cryoscopic method of molecular mass determination?
The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy.
What is cryoscopic measurement method?
Cryoscopic Method Immerse the test tube apparatus in an ice-water bath. Allow the solvent to stir continuously and equilibrate to a few degrees below the freezing point of the solvent. Record the temperature at which the solvent reaches the freezing point, which remains at a constant temperature reading.
What is the determination of molar mass?
Determining Molar Mass. We can use a measurement of any one of the following properties to determine the molar mass (molecular weight) of an unknown that is the solute in a solution: Boiling Point Elevation. Freezing Point Depression.
What is the formula for the cryoscopic method?
The cryoscopic method is one of the best methods used to determine the molecular mass of a non-volatile solute, as we have, ΔTf=Kf. m where, ΔTf is calculated experimentally and Kf is constant and by using molality we can easily find the molecular weight of the solute particle.
What is the most suitable method for determination of molecular mass?
Osmotic pressure method is best for the determination of molecular weight of polymers and protiens since osmotic pressure though very small, is measurable.
What is the cryoscopic method used to adjust?
Cryoscopic method In this method, the quantity of each substance required for an isotonic solution can be calculated from the freezing point depression values. A solution which is isotonic with blood has a of 0.52ᵒC. Therefore, the freezing point of drug solution must be adjusted to this valve.
What is the molar cryoscopic constant?
Cryoscopic constant : It is the depression of freezing point of the solvent produced on dissolving one mole of a substance in 1000 g of it. It is also called molal depression constant.
What does the cryoscopic constant depend on?
Answer: The correct option is (b) Nature of solute. The cryoscopic constant depends on the molar mass of solute molecules.
What is the meaning of cryoscopic?
cry·os·co·py krī-ˈä-skə-pē : the determination of the lowered freezing points produced in liquid by dissolved substances in order to determine molecular weights of solutes and various properties of solutions. cryoscopic. ˌkrī-ə-ˈskä-pik. adjective.
How was molar mass determined?
Molar mass is calculated by adding the atomic masses of a given compound. The periodic table provides the mass of each individual element, denoted beneath the element’s symbol. By adding the atomic masses taken from the periodic table, the molar mass can be determined.
How to determine molecular mass?
Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table. Multiply the subscript (number of atoms) times that element’s atomic mass and add the masses of all the elements in the molecule to obtain the molecular mass.
Why is the molar mass determined by measuring?
Since the magnitude of colligative property depends on the number of solute particles it is expected that the molar mass determined on the basis of colligative properties will be either higher or lower than the expected value or the normal value and is called abnormal molar mass.
What are cryoscopic measurements?
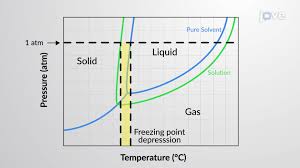
Through cryoscopy, a known constant can be used to calculate an unknown molar mass. The term “cryoscopy” means “freezing measurement” in Greek. Freezing point depression is a colligative property, so ΔT depends only on the number of solute particles dissolved, not the nature of those particles.
What is the cryoscopic method in inorganic chemistry?
Cryoscopic methods are used to determine the molecular mass of a non volatile solute. In this method, the freezing point of pure solvent is first determined. Then the freezing point of a solution (containing known amount of solute \[{W_B}\] in known amount of solvent \[{W_A}\]) is determined.
What is the cryoscopic effect?
The reduction in the freezing point of solvents due to the addition of solutes e.g. salt added to water.
What are the methods of mass determination?
For this reason, most mass determinations in the laboratory should be performed by the difference method: 1) An empty container is weighed on the balance 2) Then the chemical or object whose mass is to be determined is added to the container 3) The container now containing the chemical/object is weighed on the balance …
Which method is most accurate for the measurement of molar mass?
Measurement of osmotic pressure method is preferred for the determination of molar masses of macromolecules such as proteins and polymers.
What are the two methods of determination of molecular mass of polymers?
Thus Mn is determined by Osmometry (Osmotic pressure measurement), Ebullimetry (elevation of boiling point measurement), Cryoscopy (Freezing point depression measurement) and end group analysis.
What does the value of cryoscopic depend on?
Hence, the cryoscopic constant value depends upon the molar mass of the solute in the solution. So, the correct answer is option A. Note- Cryoscopic constant relates the molality in a solution to freezing point depression. A known constant can be used, by cryoscopy, to measure an approximate molar mass.
What is molar depression constant or cryoscopic constant?
Molal depression constant can be defined as the depression in freezing point produced when one mole of non-volatile solute is dissolved in 1 kg i.e. 1000 g of the solvent. It is also known as the cryoscopic constant.
What is freezing point depression or cryoscopic method?
With the formula below, freezing-point depression can be used to measure the degree of dissociation or the molar mass of the solute. This kind of measurement is called cryoscopy (Greek cryo = cold, scopos = observe; “observe the cold”) and relies on exact measurement of the freezing point.
How to calculate molar mass from colligative properties?
Use the freeing point depression ( Δ T f ) to calculate the molality of the solution. Then use the molality equation to calculate the moles of solute. Then divide the grams of solute by the moles to determine the molar mass.
What are cryoscopic solutions?
Solution. Cryoscopic constant or the Molal depression constant is defined as the depression in freezing point when one mole of non-volaitile solute is dissolved in one kilogram of solvent. Its unit is K.Kg.mol–1. Concept: Colligative Properties and Determination of Molar Mass – Depression of Freezing Point.
Is cryoscopic constant negative?
As you can see, the freezing-point depression cannot be negative because the van’t Hoff factor, the cryoscopic constant, and the molality of the solution are all positive values.
What is the relationship between cryoscopic constant and freezing point?
The cryoscopic constant is defined as the freezing point depression on dissolving a non-volatile solute in 1 kg of solvent. Thus, the cryoscopic constant of a liquid decreases in the freezing point when 1 mole of solute is dissolved per kg of the solvent.
What is the cryoscopic constant of benzene?
The cryoscopic constant and freezing point of benzene is 5.12 K kg mol−1 and 278.6 K respectively.
Is molarity a colligative property?
Molarity and molality are colligative properties. Q. Why is the molecular mass determined by measuring the colligative property in case of some solutes is abnormal?
What are the methods of molecular weight determination?
There are two main types of molecular weight determination: relative molecular weight determination and accurate molecular weight determination. The common determination methods include gel permeation chromatography (GPC), size exclusion chromatography (SEC), mass spectrometry, SDS-PAGE, etc.
What is the cryoscopic method in inorganic chemistry?
Cryoscopic methods are used to determine the molecular mass of a non volatile solute. In this method, the freezing point of pure solvent is first determined. Then the freezing point of a solution (containing known amount of solute \[{W_B}\] in known amount of solvent \[{W_A}\]) is determined.
What is freezing point depression or cryoscopic method?
With the formula below, freezing-point depression can be used to measure the degree of dissociation or the molar mass of the solute. This kind of measurement is called cryoscopy (Greek cryo = cold, scopos = observe; “observe the cold”) and relies on exact measurement of the freezing point.
How is molecular mass determined?
Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table. Multiply the subscript (number of atoms) times that element’s atomic mass and add the masses of all the elements in the molecule to obtain the molecular mass.
What is the purpose of the cryoscopic determination of molar mass?
Chem 3310
Fall 2020
Cryoscopic determination of molar mass
Abstract
The purpose of this experiment was to determine the molecular weight of naphthalene through the freezing point depression.
How to determine the molecular mass of a non-volatile solute?
How do you determine the molar mass of an unknown solute?
What is a cryoscopic method?
Hey there, science enthusiasts! Today, we’re diving into the fascinating world of cryoscopic determination of molar mass. This technique, also known as freezing point depression, is a powerful tool used to determine the molar mass of a solute.
But before we jump into the nitty-gritty details, let’s understand what we’re talking about. Molar mass is simply the mass of one mole of a substance. Think of it as the molecular weight, but expressed in grams per mole (g/mol). So, for example, the molar mass of water (H₂O) is 18.015 g/mol.
Now, cryoscopic determination leverages the fact that the freezing point of a solvent decreases when a solute is dissolved in it. This decrease in freezing point is directly proportional to the molality of the solute in the solution. Molality is defined as the number of moles of solute per kilogram of solvent.
Let’s break down how this method works:
1. You start with a known mass of the solvent, usually water. This is your pure solvent.
2. You carefully measure the freezing point of the pure solvent. This is your initial freezing point.
3. You then dissolve a known mass of the solute in the solvent. This creates your solution.
4. You carefully measure the freezing point of the solution. This is your final freezing point.
The difference between the initial freezing point and the final freezing point is the freezing point depression (ΔT).
Here’s the cool part. This freezing point depression (ΔT) is related to the molality of the solute by the following equation:
ΔT = Kf * m
Where:
ΔT is the freezing point depression
Kf is the cryoscopic constant of the solvent. This is a specific value for each solvent and reflects how much the freezing point will be lowered by a 1 molal solution.
m is the molality of the solute.
Now, we know the molality of the solute, but we want to find the molar mass. Let’s delve into how to calculate that:
1. You calculate the number of moles of solute using the molality and the mass of the solvent. Remember, molality is moles of solute per kilogram of solvent.
2. You calculate the molar mass by dividing the mass of the solute by the number of moles of solute.
Let’s look at a real-world example:
Imagine we want to determine the molar mass of an unknown organic compound. We dissolve 1.00 g of the compound in 100.0 g of water. The freezing point of pure water is 0.00 °C, and after adding the compound, the freezing point drops to -0.186 °C.
Here’s how we’d calculate the molar mass:
1. ΔT = 0.00 °C – (-0.186 °C) = 0.186 °C
2. Kf for water is 1.86 °C/m
3. m = ΔT / Kf = 0.186 °C / 1.86 °C/m = 0.100 m
4. Moles of solute = molality * mass of solvent (in kg) = 0.100 mol/kg * 0.100 kg = 0.0100 mol
5. Molar mass = mass of solute / moles of solute = 1.00 g / 0.0100 mol = 100 g/mol
Therefore, the molar mass of the unknown organic compound is 100 g/mol.
Cryoscopic determination is a valuable technique for chemists and researchers, especially when dealing with solutes that are difficult to analyze by other methods. It’s often used in:
Determining the molar mass of new compounds
Verifying the purity of compounds
Studying the properties of solutions
However, it’s important to be aware of certain limitations:
The technique relies on precise temperature measurements. Any errors in temperature measurement will affect the accuracy of the calculated molar mass.
The technique is most accurate for dilute solutions. As the concentration of the solute increases, the freezing point depression becomes less reliable.
The technique assumes that the solute does not dissociate or associate in solution. If the solute undergoes these reactions, the calculated molar mass will be inaccurate.
FAQs about Cryoscopic Determination of Molar Mass:
1. What are some common solvents used in cryoscopic determination?
Water is the most common solvent, but other solvents like benzene, camphor, and cyclohexane are also used depending on the solubility of the solute.
2. Why is the freezing point depression proportional to the molality of the solute?
The freezing point depression is proportional to the molality of the solute because the presence of the solute disrupts the lattice structure of the solvent during the freezing process. This disruption requires more energy to freeze the solution, leading to a lower freezing point.
3. Can I use cryoscopic determination to determine the molar mass of ionic compounds?
Yes, but you need to account for the dissociation of the ionic compound in solution. For example, if you dissolve NaCl in water, it will dissociate into Na+ and Cl- ions. This will affect the molality and therefore the freezing point depression.
4. How can I improve the accuracy of my cryoscopic determination?
You can improve the accuracy by:
* Using high-quality solvent and solute.
Calibrating your thermometer carefully.
Performing multiple measurements and taking an average.
Ensuring that the solution is well-mixed.
5. Are there any other methods to determine the molar mass?
Yes, several other methods exist, including:
Mass spectrometry
Titration
Vapor pressure osmometry
The choice of method depends on the specific compound and the available resources.
6. What are the advantages of cryoscopic determination over other methods?
* It’s a relatively simple and inexpensive method.
* It doesn’t require specialized equipment.
* It can be used for a wide range of compounds.
7. What are the disadvantages of cryoscopic determination?
* It’s less accurate than some other methods.
* It’s not suitable for all compounds.
We hope this guide has helped you understand cryoscopic determination of molar mass. Remember, practice makes perfect! So, grab your lab coat, gather your supplies, and start experimenting! You’ll be amazed at how much you can learn through this simple yet powerful technique. Happy experimenting!
See more here: What Is Cryoscopic Measurement Method? | Cryoscopic Determination Of Molar Mass
Cryoscopic determination of molar mass Lab report
The average molar mass calculated for determination 1 was 161/mol with a percent error of 26%. Determination 2 proved to be more accurate Studocu
Determination of Molar Mass from Freezing Point Depression
It is also called the cryoscopic constant of a given solvent. The depression of the freezing point is thus proportional to the molality of the solute in solution. Farmaceutická fakulta Univerzity Komenského v Bratislave
2.2: Molecular Weight Determination – Chemistry LibreTexts
For example, measured cryoscopic molecular weights of crude oil are used to predict the viscosity and surface tension for necessary fluid flow calculations in Chemistry LibreTexts
Determining Molar Mass of Solute by Cryoscopy (Instructions for
Determining Molar Mass of Solute by Cryoscopy (Instructions for a Physical Chemistry Lab Experiment) – YouTube. Physical Chemistry 101 by SciFox. 1.03K subscribers. Subscribed. YouTube
10: Determination of the Molar Mass by Freezing Point
From the experimentally determined value of m m and the mass of solute added, you can determine the molar mass of the unknown solute. The solvent that will be used in this experiment is para Chemistry LibreTexts
Cryoscopic Determination of Molecular Weight – University of
Cryoscopic Determination of Molecular Weight. This experiment shows one method of determining the molecular weight of a compound. The student will University of Scranton
Describe cryoscopic method to determine the molecular mass of
The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called Toppr
(PDF) Molar mass determination by cryoscopy: Tert
Abstract. We intend to divulge an easy experiment that permits the determination of molar masses of various compounds by cryoscopy. The major ResearchGate
Part 2. Cryoscopy | SpringerLink
Chemists, however, just saw in it one of the methods of determination of molar masses. It was only when van’t Hoff 84 gave an expression for the solvent cryoscopic constant Springer
General Chemistry I (FC, 09 – 10) Lab # 13 – Molecular Weight …
Knowing both grams and moles allows us to calculate a molecular weight. In this experiment, you will be asked to estimate the molecular weight of an unknown solute Community College of Rhode Island
See more new information: charoenmotorcycles.com
Experiment 12: Determining Molar Mass By Freezing Point Depression
Chemlab – 8. Molecular Weight Determination From Freezing Point Depression
Determining Molar Mass Of Solute By Cryoscopy (Instructions For A Physical Chemistry Lab Experiment)
Calculating Molar Mass From Freezing Point Depression
Using Freezing Point Depression To Determine Molecular Weight
Determine Molar Mass Using Freezing Point Depression | Cryoscopy (Experiment) | Colligative Property
Colligative Properties – Boiling Point Elevation, Freezing Point Depression \U0026 Osmotic Pressure
Cryoscopic Determination Of The Molecular Weight Of Unknown Substance
Cryoscopy
Link to this article: cryoscopic determination of molar mass.
See more articles in the same category here: https://charoenmotorcycles.com/how