How do you convert mol to kg m3?
What is the formula for converting density from mol/L to kg/m3? The formula for converting density from mol/L to kg/m3 is: density (kg/m3) = molar mass (g/mol) x concentration (mol/L) x 1000.
How to convert dm3 to mol?
To find the number of moles in a solution, multiply the concentration by the volume in dm3. Again, if the volume is in cm3, divide the volume by 1000 first to convert it into dm3. To find the volume of a solution, divide the moles by the concentration in mol dm–3.
What is kg/dm3?
Kilogram Per Cubic Decimeter (kg/dm3) is a unit in the category of Density. It is also known as kilograms per cubic decimeter, kilogram per cubic decimetre, kilograms per cubic decimetre, kilogram/cubic decimeter, kilogram/cubic decimetre.
How many moles are in 1 kg?
Explanation: The idea here is that 1 kg-mole is equal to 103 moles. This is the case because a mole of a substance must contain a number of particles of that substance equal to the number of atoms present in exactly 12 g of carbon-12.
What is mol per dm3?
The units moles per liter (mol liter–1) or moles per cubic decimeter (mol dm–3) are used to express molar concentration. They are equivalent (since 1 dm–3 = 1 liter). If a pure substance is soluble in water, it is easy to prepare a solution of known concentration.
Is molarity the same as mol dm3?
In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol/dm3 in SI units. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M or 1 M.
Is mol dm 3 volume?
The units of concentration are mol/dm3. This is because moles have the unit ‘mol’, and volume is measured in ‘dm3‘. Practice Question: Harry has 250 cm3 solution in a beaker containing 4 moles of calcium carbonate. Calculate the concentration of the solution.
Is 1M the same as 1 mol Dm3?
We usually measure the concentration of a solution in moles per dm3 (mol dm–3). The old term ‘Molar’ is sometimes seen on solutions – a 1 Molar solution (1M) contains 1 mole of solute per dm3 of solvent.
What is 24 mol per Dm3?
1 mole of gas occupies 24 dm3. If we have one mole of gas at room temperature, then it has a volume of 24 dm3. This volume is also known as the molar gas volume, applying to all gases.
How to convert mol to kg?
Re: Molar Mass to Kg From mol you would multiply by avogadro’s number which would be 6.0221 x 10^23 and then multiply by 10^3 to convert from g to kg.
How to convert cm3 to kg?
1 g/cm3 is equivalent to 1000 kg/cubic meter. To convert 100 gram to kilogram, divide it by 1000, 100/1000 = 0.1 kg. To convert any gm/cm3 amount to kg/m3, multiply it by 1000. This is how the conversion of 1g cm3 to kg m3 is done through the formula.
What is 1 kg m3 equal to?
1 kg is 1000 grams. 1 cubic meter (a cube with side length 1 m) is made up of 1000 cubic decimeters (cubes with 1 dm sides). So if you have 1000 grams per 1 m3 , it is the same as 1000 grams per 1000 cubic decimeters (or 1000 liters). So 1kg/m3 = 1000 g/1000 dm3 = 1000 g/1000 L = 1 g / L.
How do you calculate kg m3?
The density of an object or substance can be calculated from this equation: density in kilograms per meter cubed is equal to mass in kilograms, divided by volume in meters cubed (p = m / v).
How to convert volume into kg?
let’s say you have a volume of 2 liters of water. The density of water is approximately 1000 kg/m^3, so you would use the formula like this:mass (kg) = 2 liters x 1000 kg/m^3 = 2 x 0.001 m^3 x 1000 kg/m^3 = 2 kgKeep in mind that the density of a material can vary depending on factors such as temperature and pressure.
How much kg is 1 mole?
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in exactly 12 g (or 0.012 kg) of the carbon-12 isotope.
What is mol per kg?
A commonly used unit for molality is the moles per kilogram (mol/kg). A solution of concentration 1 mol/kg is also sometimes denoted as 1 molal. The unit mol/kg requires that molar mass be expressed in kg/mol, instead of the usual g/mol or kg/kmol.
What is 22.4 mol Dm3?
The molar volume of a gas at STP is 22.4 dm³/mol or 22.4 L/mol. STP in this case is defined as 1 atm and 0 °C (32 °C, 273.15 K). At STP, water is in its solid form, not a gas.
Is M equal to mol Dm3?
Concentration of solutions is measured in moles per decimetre cubed (mol dm–3), often abbreviated to molar or M. This is the number of moles of solute contained in one decimetre cubed of solution.
What is 24 Dm3 per mole?
The molar volume close molar volumeThe volume occupied by one mole of any gas (24 dm³ or 24000 cm³ at room temperature and pressure). is the volume occupied by one mole of any gas, at room temperature and pressure. The molar volume is equal to 24 dm3 (24,000 cm3). This volume is given in questions that need it.
Can you convert molarity to moles?
If you multiply the equation by volume you have a way to solve for number of moles, or MV = n. So basically you can multiply the molarity by volume to find the number of moles of solute. Then you can use the molar mass of the solute to convert moles of solute into grams of solute, or the mass. Hope that helps.
What is dm3 equal to in chemistry?
Dm3 may refer to: Cubic decimetre ( ), a volume unit which is exactly equivalent to a litre.
How do you convert m cubed to kg?
1 cubic meter of water is 1,000 kilograms. So in your case you’d simply multiply volume times 1,000.
How many moles are in a m3?
1 gm mole of a gas at STP occupies 22.4 litres. The number of molecules of the gas at STP is 6.023 x 10^23 (Avogadro number). Therefore, at STP, 1000 litres (1 m^3) of space will contain 1000/22.4 moles of the ideal gas, or: 6.023 x 10^23 x 1000/22.4 = 2.689 x 10^25 molecules.
What is 1 mol per m3?
One mole is equal to approximately 6.022169 x 10 23 , and is also called the Avogadro constant. Thus, 1 mol / m 3 represents approximately 6.022169 x 10 23 atoms of a substance in one cubic meter of space.
How do you convert molecular mass to kg?
To convert molar mass from grams per mole (g/mol) to kilograms per mole (kg/mol), you need to divide the value in grams by 1000.
What is g/dm3 in kg/m3?
How to convert kilograms per cubic decimeter to kg/m3?
How to convert mol/L to kg/m3?
How many kg/m3 in 1 lb/ft 3?
Understanding the Units
First things first, we need to understand what these units represent.
mol dm3 stands for moles per cubic decimeter. A mole is a unit of amount, basically a specific number of particles (like molecules or atoms). A decimeter (dm) is a unit of length, equal to 1/10 of a meter. So, mol dm3 represents the concentration of a substance in moles per unit volume.
kg m3 stands for kilograms per cubic meter. A kilogram (kg) is a unit of mass, and a meter (m) is a unit of length. So, kg m3 represents the density of a substance in kilograms per unit volume.
The Conversion Process
Converting mol dm3 to kg m3 involves a few steps. It’s basically about finding the mass of a specific number of moles and then relating it to the volume.
1. Molar Mass
We need to know the molar mass of the substance we’re working with. The molar mass is the mass of one mole of that substance, usually expressed in grams per mole (g/mol). You can find this information on the periodic table.
2. Density Calculation
Now, let’s say we have a concentration of X mol dm3. To convert this to kg m3, we need to:
Convert dm3 to m3: 1 cubic decimeter (dm3) is equal to 1/1000 cubic meters (m3).
Calculate the mass: Multiply the concentration (X mol dm3) by the molar mass (in g/mol) and then by 1000 to get the mass in kilograms per cubic meter (kg/m3).
Example
Let’s say we have a solution of glucose with a concentration of 0.5 mol dm3. Here’s how we convert it to kg m3:
1. Molar Mass of Glucose: The molar mass of glucose (C6H12O6) is approximately 180 g/mol.
2. Conversion:
* 0.5 mol dm3 * 180 g/mol * 1000 g/kg * (1 dm3 / 1000 m3) = 90 kg m3
Let’s Summarize
To convert from mol dm3 to kg m3, you need to:
1. Know the molar mass of the substance.
2. Convert dm3 to m3 (multiply by 1000).
3. Calculate the mass by multiplying the concentration by the molar mass and then by 1000.
Remember: This conversion process relies on the relationship between molar mass, concentration, and density.
FAQs
Q1: Why is this conversion important?
A: This conversion is important in many fields, including chemistry, engineering, and materials science. It allows us to understand the mass of a substance within a specific volume, which is crucial for many applications.
Q2: What if I have the concentration in mol/L?
A: The conversion process is very similar. A liter (L) is equal to a cubic decimeter (dm3), so mol/L is equivalent to mol dm3. You can apply the same steps we discussed earlier.
Q3: Can you give me another example?
A: Let’s take sodium chloride (NaCl) with a concentration of 1.2 mol dm3.
Molar Mass of NaCl: 58.44 g/mol
Conversion:
* 1.2 mol dm3 * 58.44 g/mol * 1000 g/kg * (1 dm3 / 1000 m3) = 69.73 kg m3
Q4: Is there an online tool for this conversion?
A: Yes, you can find online tools and calculators specifically designed for this conversion. Just search for “mol dm3 to kg m3 converter” on your preferred search engine.
I hope this explanation helps you understand the conversion from mol dm3 to kg m3.
See more here: How Do You Convert Mol Dm 3 To G Dm 3? | Mol Dm3 To Kg M3
Conversion g/dm^3 to kg/m^3, g/dm3 to kg/m3, g/dm^3 to
The conversion factor is 1; so 1 gram per cubic decimeter = 1 kilogram per cubic meter. In other words, the value in g/dm 3 multiply by 1 to get a value in kg/m 3. The conversion factor is 1, i.e., the volume given in g/dm 3 is numerically equal to that expressed in kg/m 3. HackMath
How to convert density from mol/L to kg/m3? – Physics Forums
The formula for converting density from mol/L to kg/m3 is: density (kg/m3) = molar mass (g/mol) x concentration (mol/L) x 1000. 2. How do I convert from mol/L to Physics Forums
Concentration – Molar Converter
Free online concentration – molar converter – converts between 12 units of concentration – molar, including mol/cubic meter [mol/m^3], mol/liter [mol/L], mol/cubic centimeter, Unit Converter
Density Converter
Free online density converter – converts between 42 units of density, including kilogram/cubic meter, gram/cubic centimeter, kilogram/cubic centimeter, gram/cubic Unit Converter
Molar Volume Unit Converter
7 rows Abbreviation Unit; m3/mol: cubic metre per gram-mole: L/kmol: litre per kilogram-mole: L/mol: litre per gram-mole: dm3/kmol: cubic decimetre per kilogram-mole Unit Converter App
kg/dm³ to kg/m³ conversion tables with examples and formulas
21 rows How to convert kilograms per cubic decimeter to kilograms per cubic meter [kg/dm³ to kg/m³]: ρ kg/m³ = 1 000 × ρ kg/dm³. How many kilograms per cubic meter in Aqua-Calc
Density Conversion Calculator
To simply convert from any unit into kg/m 3, for example, from 50 lb/ft 3, just multiply by the value in the right column in the table below. 50 lb/ft 3 * 16.018463 [ (kg/m 3) / (lb/ft 3) ] = 800.92315 kg/m 3 Calculator Soup
Density Converter – The Engineering ToolBox
Online density converter with commonly used units. Density is an expression of the ratio of mass per unit volume . The symbol used for density is rho, ρ . ρ = m / V (1) where. ρ = density (kg/m3, lbm /ft3 …) m The Engineering ToolBox
kg/dm³ to kg/m³ | Kilogram per Cubic Decimeter to kg/m³
When you are converting density from Kilogram per Cubic Decimeter to kg/m³, you need a converter that is elaborate and still easy to use. All you have to do is select the unit for Units Converters
See more new information: charoenmotorcycles.com
How To Convert G/Cm3 To Kg/M3 (And Never Be Wrong Again)
G/Cm^3 To Kg/M^3 (Unit Conversions)
How To Convert Mol/Dm³ To G/Dm³ – Gcse Chemistry | Kayscience.Com
Cubic Unit Factor Label Conversions (G/Cm3 To Kg/M3)
Conversion Between Different Units Of Volume
Convert G/Ml Into Kg/M3 (Units)
Convert G/Ml To Kg/M3
Conversión De Kilogramo Por Metro Cubico Kilogramo Por Litro – Kg/M3 A Kg/L
Kilogramos Por Metros Cúbicos A Gramos Por Mililitros (Kg/M3 A G/Ml)
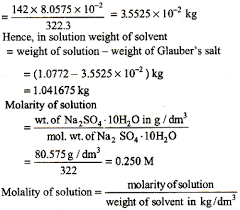
Link to this article: mol dm3 to kg m3.
See more articles in the same category here: https://charoenmotorcycles.com/how