What forces are broken during vaporization?
During vaporization, water molecules gain energy greater than a certain threshold level such that it can break the weak intermolecular forces or bonds or the hydrogen bond and escape as water vapors. So we can say vaporization a process of breaking the hydrogen bonds between the water molecules and making them free.
When water evaporates what attraction is broken?
Hydrogen bonding is an attraction between the hydrogen of one water molecule to the lone pairs of oxygen of another water molecule. Thus hydrogen bonds are broken during vaporization, but not the covalent bonds between hydrogen and oxygen within a molecule.
Which type of interaction is broken as water vaporizes?
Heat of vaporization of water Just as it takes a lot of heat to increase the temperature of liquid water, it also takes an unusual amount of heat to vaporize a given amount of water, because hydrogen bonds must be broken in order for the molecules to fly off as gas.
What bonds break during vaporization?
The fact that hydrogen bonds need to be broken for water to evaporate means that a substantial amount of energy is used in the process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place.
What type of attraction is broken when water vaporizes?
Answer and Explanation: The correct answer is c) hydrogen bonds. Hydrogen bonds, must be broken for water to vaporize. These bonds form due to the interaction between the hydrogen atom of water (directly connected to oxygen, which is a highly electronegative atom) and the oxygen atom.
Which type of bond must be broken for water to vaporize *?
Hydrogen bonds must be broken for water to evaporate. Water molecules with high kinetic energy evaporate; remaining molecules are cooler.
Which is broken when water evaporates?
How does water evaporate? To make water evaporate, energy has to be added. The water molecules in the water absorb that energy individually. Due to this absorption of energy the hydrogen bonds connecting water molecules to one another will break.
Are intramolecular forces broken during evaporation?
When a substance is evaporating, the intermolecular forces will be broken, while the intramolecular forces will remain intact. For example, when liquid water turns to water vapor, the hydrogen bonds between different H2O molecules are broken, but the H-O bonds within each molecule remain intact. Hope this helped!
When a substance evaporates, what type of force is broken?
Understanding the Evaporation Process Evaporation is a phase transition from the liquid phase to gas phase. This process involves the breaking of intermolecular forces between water molecules, such as hydrogen bonds.
What happens when water vaporizes?
Evaporation occurs when a liquid changes to a gas. During evaporation, molecules gain enough thermal energy to escape from the liquid surface into the air as water vapor. Water-vapor molecules spread out, breaking completely free of each other, and then disperse or spread out among the other gas molecules in the air.
What is it called when water vaporizes?
Evaporation is the process that changes liquid water to gaseous water (water vapor).
What bonding is broken when you turn water into steam?
Answer and Explanation: When the water reaches its boiling point, it begins to evaporate. When the water gets converted to steam, the bonds that break are the covalent bonds and the hydrogen bonds.
Do intermolecular forces break during vaporization?
Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid.
Which bonds or forces are broken when ethanol is vaporized?
Figure 4.4 Ethanol vaporizes when its hydrogen bonds are broken. This happens more easily compared to water because ethanol molecules are bound to each other with up to three hydrogen bonds, while water molecules are bound to each other with up to four hydrogen bonds.
What bond is broken when water melts?
The hydrogen bonds between water molecules in ice produce the open structure shown in the figure below. When ice melts, some of these bonds are broken, and this structure collapses to form a liquid that is about 10% denser. This unusual property of water has several important consequences.
Do bonds break during evaporation?
No new compound is formed and the molecule is still the same. Thus, no bonds within a molecule are broken. Instead, the intermolecular forces of attraction between the liquid molecules are broken or disrupted, causing the transformation into gas.
When water vaporizes, which type of attraction intermolecular or intramolecular is broken?
Explanation: In order for water to evaporate, its molecules must have enough kinetic energy to allow them to overcome the relatively strong intermolecular forces (IMFs) of attraction that keeps the molecule together in the liquid phase.
Which types of chemical bonds are easily broken in water?
Every water molecule can be hydrogen bonded with up to three other water molecules (See Fig. 3-7). However, because hydrogen bonds are weaker than covalent bonds, in liquid water they form, break, and reform easily.
What bond is broken by water?
A typical configuration of liquid water has many instances of water molecules with apparently broken hydrogen bonds.
What bonds can water break?
In its liquid state, water molecules cling to one another through so-called hydrogen bonding, constantly making and breaking bonds as they jumble about.
Are intramolecular bonds broken during vaporization?
Explanation: Intramolecular bonds are not broken during vapourisation of water. Intramolecular bonds are bonds that exist within a molecule, whilst intermolecular bonds are bonds that exist between different molecules.
What type of bond must be broken for water to vaporize?
In order for water to evaporate, hydrogen bonds must be broken. Water’s heat of vaporization is 540 cal/g.
What type of bond is broken when water changes from liquid to gas?
Key Points. As water is boiled, kinetic energy causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapor).
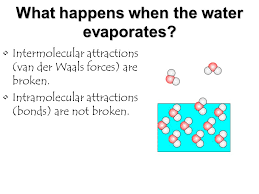
What happens when water evaporates?
Evaporation is a key step in the water cycle. Evaporation happens when a liquid turns into a gas. It can be easily visualized when rain puddles “disappear” on a hot day or when wet clothes dry in the sun. In these examples, the liquid water is not actually vanishing—it is evaporating into a gas, called water vapor.
What forces are being broken when water molecules evaporate?
This involves the breaking of intermolecular bonds, which are the forces of attraction between separate water molecules, and not the intramolecular bonds within the water molecule itself. This process requires energy to overcome these forces of attraction, ultimately allowing the water molecules to escape as gas.
Does water break intermolecular forces?
Because the forces between molecules are strong, water has to be heated to 100 ℃ before it changes phase. At this temperature, the molecules have enough energy to break the intermolecular forces that hold the molecules together.
Can intramolecular bonds be broken?
Intramolecular bonds form between atoms in a molecule. These bonds are very strong and take a lot of energy to break. Breaking these bonds results in the loss of identity of the molecule. For example, if you break the bonds between hydrogen and oxygen atoms in the water molecule, you no longer have water.
Do intermolecular forces break during vaporization?
Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid.
What forces oppose vaporization?
Hydrogen bond attraction opposes vaporization.
When a substance evaporates, what type of force is broken?
Understanding the Evaporation Process Evaporation is a phase transition from the liquid phase to gas phase. This process involves the breaking of intermolecular forces between water molecules, such as hydrogen bonds.
What type of forces are overcome when liquid water is vaporized?
Yes, the intermolecular forces are overcome when liquid water is vaporized. Intramolecular forces are overcome when gaseous water is converted to hydrogen gas and oxygen gas.
What causes hydrogen bonds to break in water?
How do water molecules evaporate?
How do water molecules overcome intermolecular forces?
Why do water molecules vibrate when H-O bonds are stretched or bent?
It’s All About Hydrogen Bonds!
When water changes from a liquid to a gas (we call that vaporizing or boiling), hydrogen bonds are the attractions that break. Think of them as the little “glue” holding water molecules together.
Here’s a Breakdown:
Water molecules (H2O) are made up of two hydrogen atoms and one oxygen atom.
Hydrogen bonds are special attractions that happen between the hydrogen atom of one water molecule and the oxygen atom of a neighboring water molecule.
* These hydrogen bonds are stronger than typical attractions between molecules, which is why water is a liquid at room temperature.
Imagine It Like This:
Picture water molecules like tiny magnets. The positive end of one magnet (the hydrogen atom) is attracted to the negative end of another magnet (the oxygen atom). These attractions are the hydrogen bonds.
What Happens When Water Vaporizes?
When you heat water, you’re giving those water molecules more energy. This extra energy causes the molecules to move faster and vibrate more. The increased movement eventually overcomes the hydrogen bonds holding them together.
The “Glue” Breaks, and the Molecules Escape
With the hydrogen bonds broken, the water molecules are free to escape the liquid and become a gas, or steam. That’s why you see bubbles forming when water boils – it’s those escaping water molecules.
Let’s Summarize:
* Water molecules are held together by hydrogen bonds.
* These bonds are stronger than typical intermolecular attractions.
* When you heat water, the molecules gain energy, vibrate more, and eventually break the hydrogen bonds.
Vaporization is the process of water changing from a liquid to a gas, where the molecules escape due to the broken hydrogen bonds.
Why Does This Matter?
Understanding hydrogen bonds is important because it helps us understand:
Why water has a relatively high boiling point. Breaking those strong bonds takes more energy than breaking weaker attractions between other molecules.
How water’s properties make it essential for life. Water’s ability to form hydrogen bonds makes it a great solvent, and allows for many important biological processes.
Let’s Look at Some FAQs About Water Vaporization and Hydrogen Bonds:
FAQs
What are the differences between hydrogen bonds and covalent bonds?
Great question! Covalent bonds are the forces that hold atoms together *within* a molecule. For example, the covalent bonds between hydrogen and oxygen atoms create the water molecule (H2O). Hydrogen bonds are attractions *between* molecules, like the attraction between one water molecule and another.
Why do hydrogen bonds only form between specific atoms?
Hydrogen bonds are a bit picky about who they hang out with. They usually form between hydrogen atoms that are bonded to a highly electronegative atom (like oxygen or nitrogen) in one molecule, and an electronegative atom in a neighboring molecule. This difference in electronegativity creates a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom, allowing for the attraction.
What other substances have hydrogen bonds?
Lots of things have hydrogen bonds, especially things containing oxygen or nitrogen. For example, DNA, proteins, and even sugar molecules use hydrogen bonds. They are really important for life!
How does vaporization differ from evaporation?
Evaporation is a slower process that occurs at the surface of a liquid, even at temperatures below the boiling point. Vaporization is a faster process that occurs when a liquid reaches its boiling point and changes to a gas.
I hope this information helps you understand what’s going on when water vaporizes. If you have any more questions, feel free to ask!
See more here: When Water Evaporates What Attraction Is Broken? | When Water Vaporizes Which Type Of Attraction Is Broken
What Intermolecular Forces are Present in Water? | Sciencing
The H 2 O water molecule is polar with intermolecular dipole-dipole hydrogen bonds. As the water molecules attract each other and form bonds, water Sciencing
Specific heat, heat of vaporization, and density of water – Khan
When the heat is raised (for instance, as water is boiled), the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allows water Khan Academy
Intermolecular Forces – Division of Chemical Education, Purdue
There are three ways in which a water molecule move: (1) vibration, (2) rotation, and (3) translation. Water molecules vibrate when H–O bonds are stretched or bent. Rotation Division of Chemical Education
Intermolecular forces and vapor pressure (video) | Khan Academy
On the other hand, things with high intermolecular forces, fewer of those molecules are going to break away, and so you’re going to have a lower vapor pressure when you get to that Khan Academy
What is needed for evaporation to occur? Explain in terms of
Explanation: In order for water to evaporate, its molecules must have enough kinetic energy to allow them to overcome the relatively strong intermolecular forces Socratic
8.1: Intermolecular Interactions – Chemistry LibreTexts
London-dispersion, dipole-dipole, and hydrogen-bonding forces of attraction are being overcome to separate the liquid water molecules into gaseous water molecules. (Covalent bonds bind oxygen to the two Chemistry LibreTexts
12.7: Intermolecular forces – Chemistry LibreTexts
For example, it requires 927 kJ to overcome the intramolecular forces and break both O–H bonds in 1 mol of water, but it takes only about 41 kJ to overcome the intermolecular attractions and Chemistry LibreTexts
12.4: Evaporation and Condensation – Chemistry LibreTexts
As some water molecules become vapor, an equal number of water vapor molecules condense back into the liquid state. Condensation is the change of state from a gas to a Chemistry LibreTexts
When water evaporates are intermolecular or intramolecular
When water evaporates, intermolecular bonds between water molecules are broken, not intramolecular bonds within the water molecule itself. The intermolecular Answers
See more new information: charoenmotorcycles.com
What Type Of Bonds Are Broken When Water Evaporates?
When Water Boils What Forces Are Being Broken?
What Type Of Bonds Are Broken When Water Evaporates?
How Polarity Makes Water Behave Strangely – Christina Kleinberg
Properties Of Water
Heating Matter And Changes In State
Vapor Pressure Explained
Intermolecular Forces And Boiling Points
Attraction Of Water Molecules
Link to this article: when water vaporizes which type of attraction is broken.
See more articles in the same category here: https://charoenmotorcycles.com/how